The ENACT Network
As part of our suite of multisite clinical trial support, CCTS has access to the CTSA-supported ENACT Network. From cohort discovery to assistance with a SMART IRB, CCTS can faciliate the necessary connections to expand your clinical study's reach.
What is the ENACT Network? Heading link

The ENACT Network is a federated data network of leading academic medical centers in the CTSA consortium. This HIPAA-compliant and IRB-approved cohort discovery tool was developed collaboratively by members of NCATS’ Clinical and Translational Science Award (CTSA) consortium, with funding from the NIH National Center for Advancing Translational Sciences.
ENACT builds upon the CTSA Consortium cohort discovery platform, ACT, to enable investigators and trainees at CTSA hubs to conduct EHR-based research on any disease or condition across the network of over 142M patients.
What institutions are in the ENACT Network? Heading link
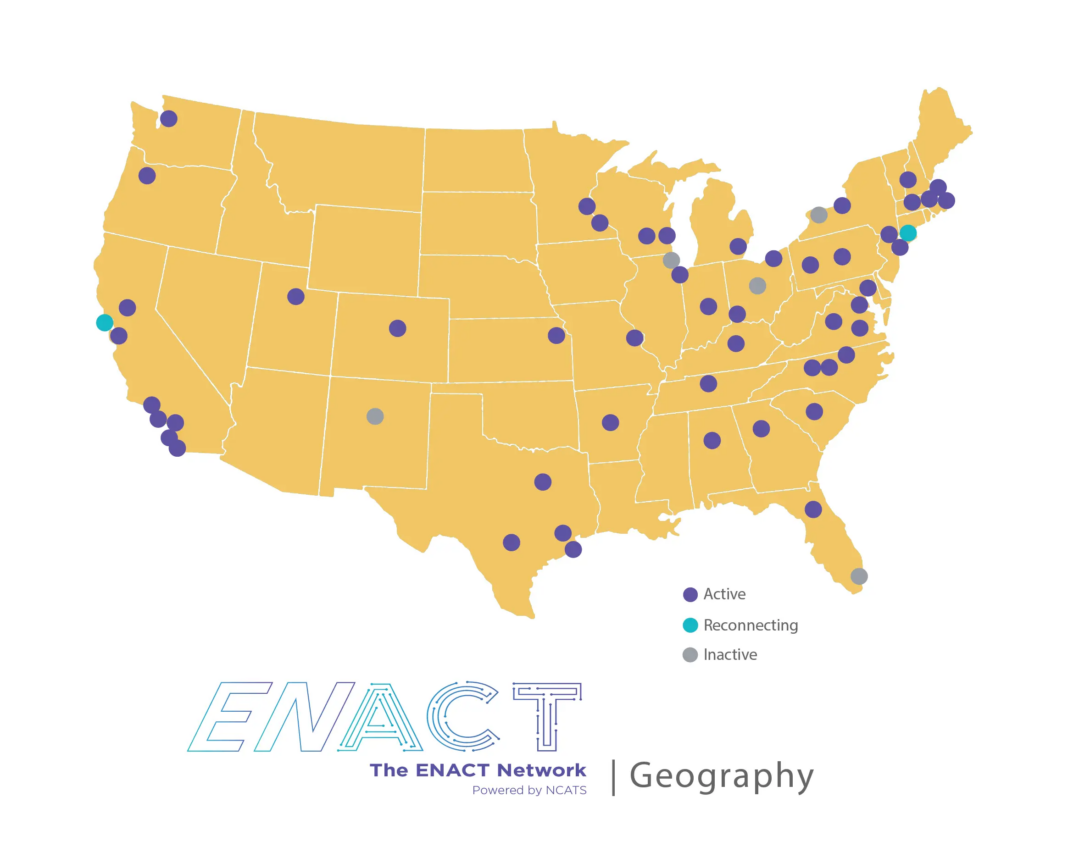
Click here to see which institutions have joined ENACT, and which institutions will join in future waves. Ultimately, ENACT plans to include all institutions that are part of the NIH-funded Clinical and Translational Science Award (CTSA) program.
CTSA hub partner sites, Institutional Development Award (IdEA) sites, and Research Center in Minority Institutions (RCMI) are also invited to participate in ENACT.
What can I do with ENACT? Heading link
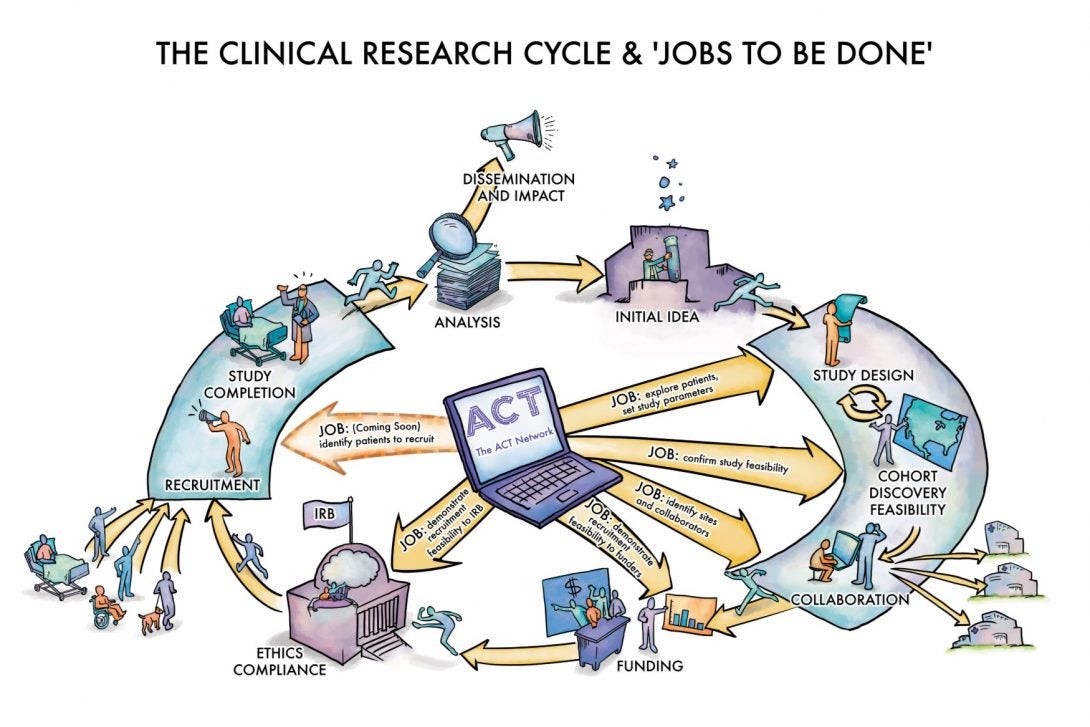
The ACT Network helped clinical investigators conduct cohort discovery before a trial starts, to establish feasibility of a clinical protocol for grant applications, IRB submission, etc. It also helped investigators identify additional sites for clinical trials. By allowing investigators to thoroughly explore patient cohorts and potential sites before finalizing their clinical protocols, ACT increased the odds of successfully completing clinical trial recruitment.
ENACT will build upon the initial ACT Network framework to expand the ability to conduct EHR research, introduce data quality standards, and make available new informatics tools. It also provides a network for investigators to leverage for other funding opportunities.
Available Data:
The ENACT Network queries the total numbers of patients at each participating site meeting your inclusion or exclusion criteria for demographics (age, gender, race, etc.), diagnoses (ICD9/10 codes), lab results, and most frequently prescribed medications. Not all data points are available for all patients.
View the current ENACT Network Ontology & Data Dictionary
Additional data elements are added on an annual basis to stay current with investigator needs. New data elements for 2023 include updates to social determinants of health and additional labs.
How can I access ENACT? Heading link
Interested users can request an ENACT Network account by contacting Joanna Tess at jtess2@uic.edu.

CTSA Trial Innovation Network Heading link
The TIN is a collaborative national network that focuses on operational innovation, operational excellence, and collaboration and leverages the expertise and resources of the CTSA Program.