“Research Billing Review & Set-Up Process”
Best Practice Hour Seminar Series
September 20, 2022
12:00 PM - 1:00 PM
Calendar
Download iCal File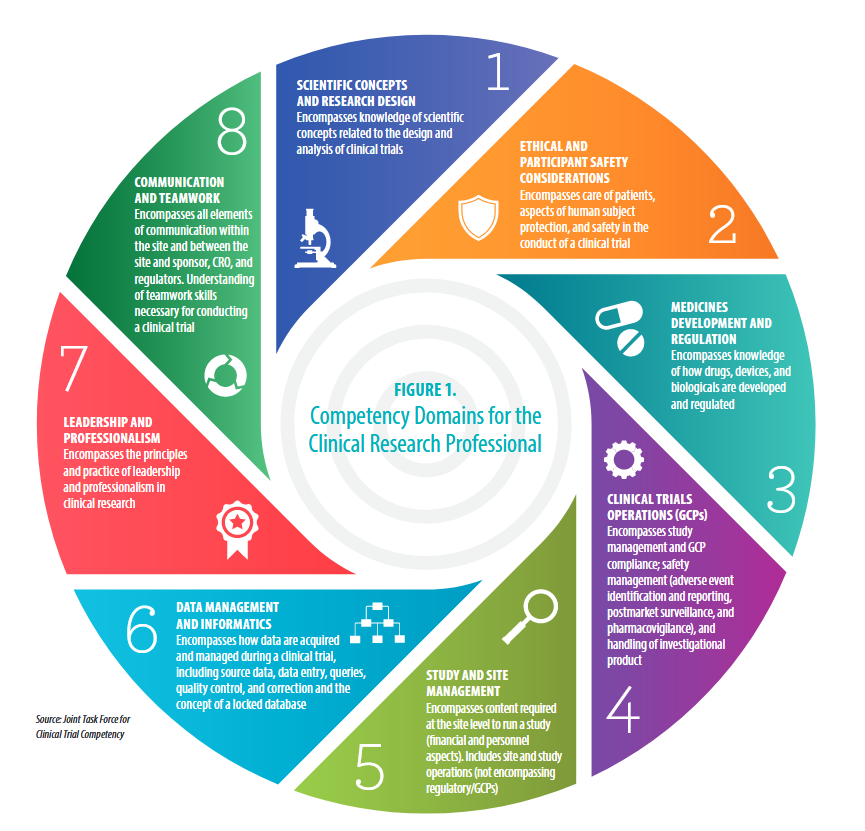
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
This presentation will cover:
- Getting Your Study into Epic
- Linking Patients and Keeping Enrollment Status/Study Status Up to Date to Assure Correct Billing
- How Research Charges Are Processed
Speaker:
Scott Kennedy
Assistant Director, Clinical Research Finance Office
Competency Area(s):
#5 - Study and site management: Site and study operations
Date posted
Jul 26, 2022
Date updated
Sep 1, 2022