“How to submit to the IRB”
Best Practice Hour Seminar Series
February 20, 2024
12:00 PM - 1:00 PM
Location
Virtual via Zoom
Calendar
Download iCal File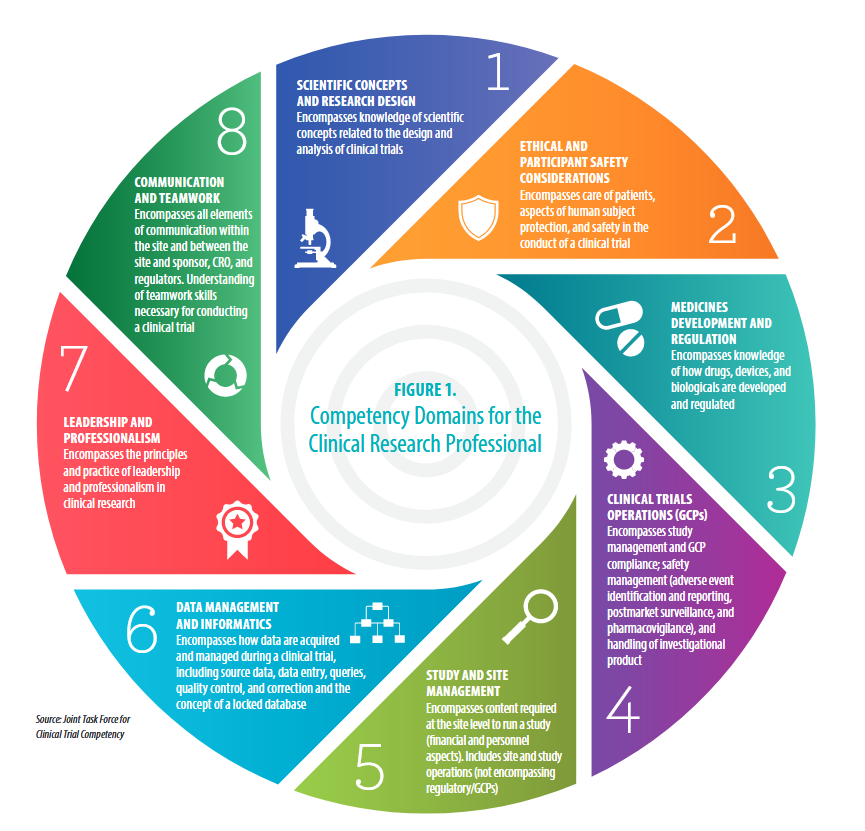
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
This presentation will give an overview of the steps to submitting a new research project to the IRB. It will cover the types of projects that require a submission to OPRS or the IRB, how to figure out what type of review is needed, and the steps to prepare and submit a project. Tips to make the process more successful and links to resources will be provided.
Speaker:
Rachel Olech
Senior Regulatory Specialist, CCTS
Competency Area:
#2: Ethical and Participant Safety Considerations
Register for Zoom login details
Date posted
Jan 22, 2024
Date updated
Jan 29, 2024