“An Overview of the Contract Review Process for Industry Sponsored Clinical Trials”
Best Practice Hour Seminar Series
July 16, 2024
12:00 PM - 1:00 PM
Location
Virtual via Zoom
Calendar
Download iCal File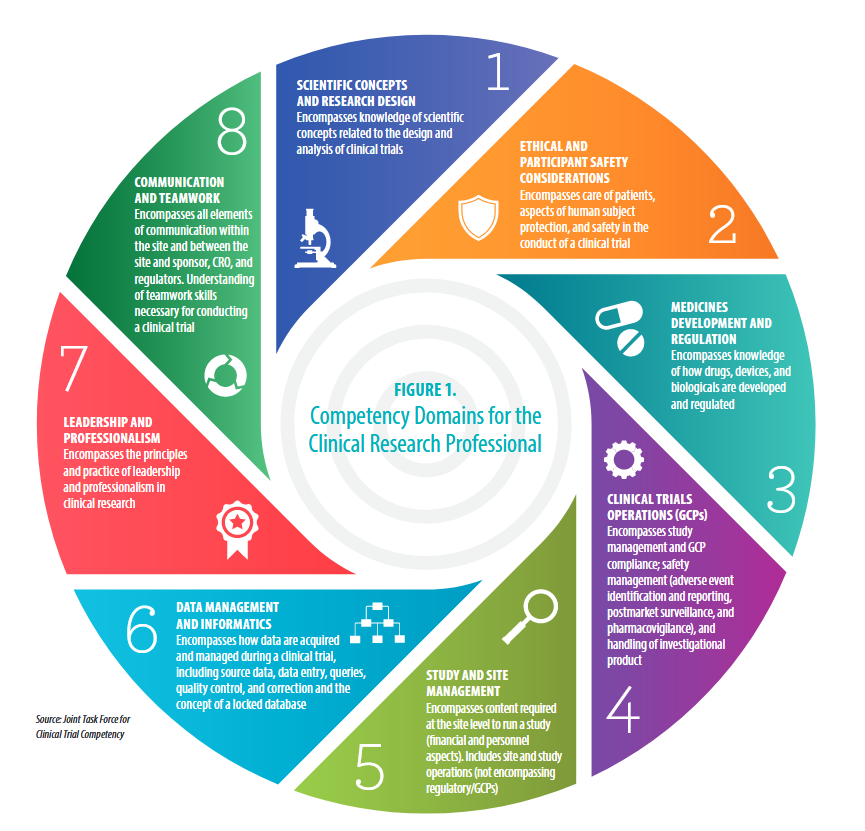
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
This session will provide insight into key provisions of Industry Sponsored Clinical Trial Agreements (publication, confidentiality, intellectual property, subject injury reimbursement, and indemnification). Learn how the Research Administrator can facilitate the review process and help prevent delays.
Speaker:
Brenda Barrie, CRA
Clinical Research Contracts Specialist
Office of Sponsored Programs, UIC
Competency Area:
#5: Study and Site Management
Register for Zoom login details
Date posted
Mar 4, 2024
Date updated
Apr 16, 2024