“Informed Consent & Questionnaires of Non-English Speaking Participants and Special Populations”
Best Practice Hour Seminar Series
October 15, 2024
12:00 PM - 1:00 PM
Location
Virtual via Zoom
Calendar
Download iCal File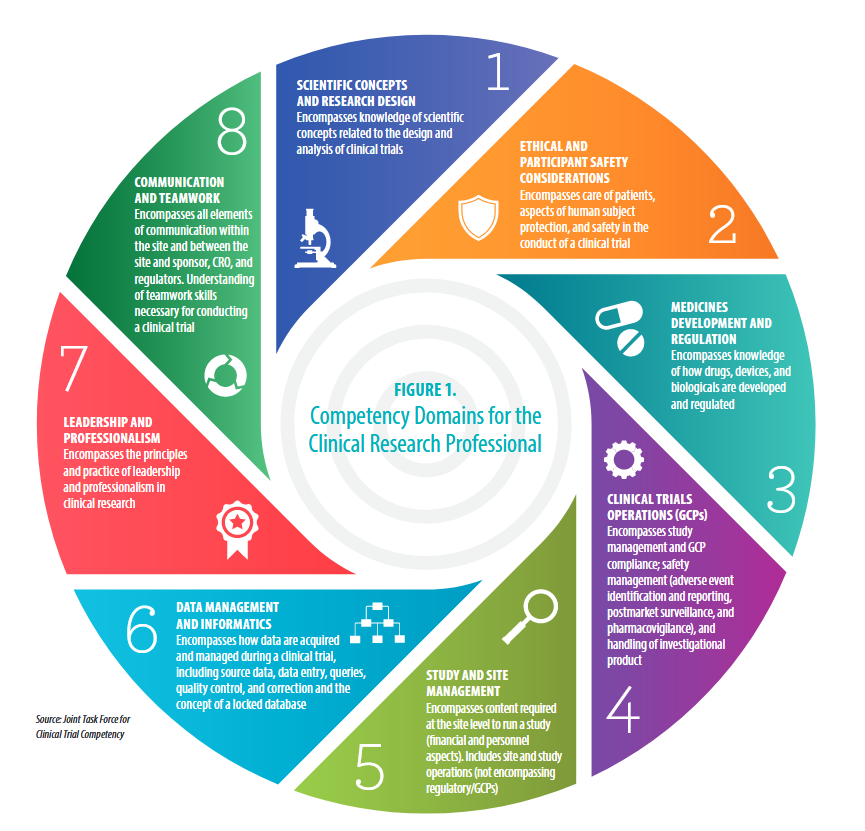
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
This seminar will cover the policy, regulatory requirements, and patient facing aspects of consenting non-English speaking patients.
Speaker:
Rachel Olech
Senior Regulatory Specialist, CCTS
&
Meredith Russell
Associate Director of Clinical Research Operations
Clinical Trials Office, UI Cancer Center
Competency Area:
#2: Ethical and Participant Safety Considerations
Register for Zoom login details
Date posted
Sep 4, 2024
Date updated
Sep 4, 2024