“Adverse Events/Serious Adverse Events & Unanticipated Problems Identification & Documentation”
Best Practice Hour Seminar Series
October 17, 2023
12:00 PM - 1:00 PM
Location
Virtual via Zoom
Calendar
Download iCal File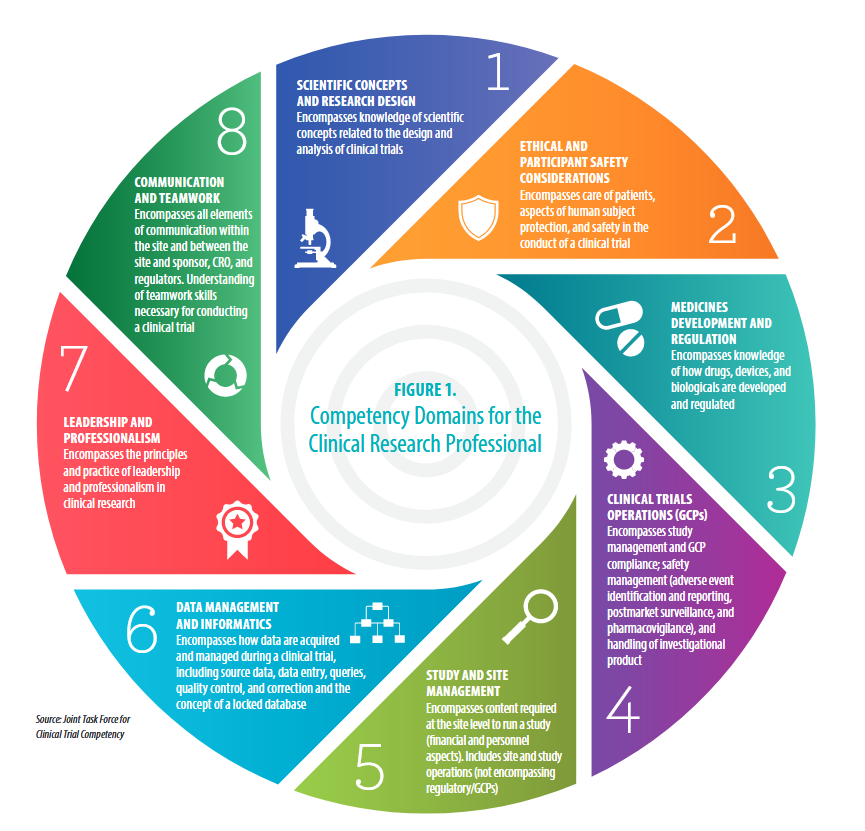
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
The Best Practice Hour (BPH) is a series sponsored by the University of Illinois Cancer Center and the UIC Center for Clinical and Translational Science. Each session will explore best practices for one aspect of conducting human subjects research (HSR).
OBJECTIVES:
- Identify how GCPs protect research participants
- Define adverse event (AE)
- Define criteria for a serious adverse event (SAE)
- Specify Research team members’ responsibilities for managing AE/SAE
- Identify criteria for Prompt Reporting to the IRB
- Identify minimum criteria for SAE reporting to the FDA and/or sponsor
Speaker:
Annette Kinsella, RN, CCRC
QA Education Specialist
UI Cancer Center, UIC
Competency Area:
#4: Clinical Trial Operations
Register for Zoom login details
Date posted
Sep 7, 2023
Date updated
Sep 7, 2023