“Informed Consent for Special Populations”
Best Practice Hour Seminar Series
May 16, 2023
12:00 PM - 1:00 PM
Calendar
Download iCal File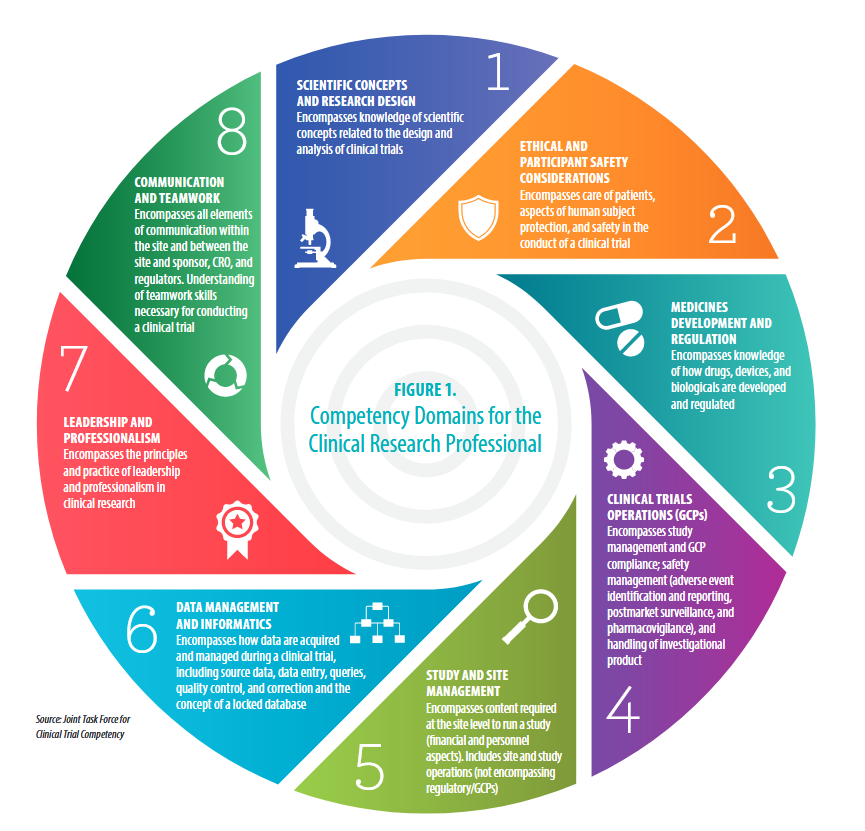
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
This presentation will cover special topics when obtaining informed consent, including identifying circumstances that may arise during the consent process for certain populations, how to address these circumstances, and regulatory issues specific to these populations.
Speaker:
University of Illinois Cancer Center Clinical Trials Office
Competency Area:
#2: Ethical and Participant Safety Considerations
Date posted
Feb 10, 2023
Date updated
Nov 14, 2024