“Top 10 Reasons for Modifications from the IRB”
Best Practice Hour Seminar Series
January 21, 2025
12:00 PM - 1:00 PM
Location
Virtual via Zoom
Calendar
Download iCal File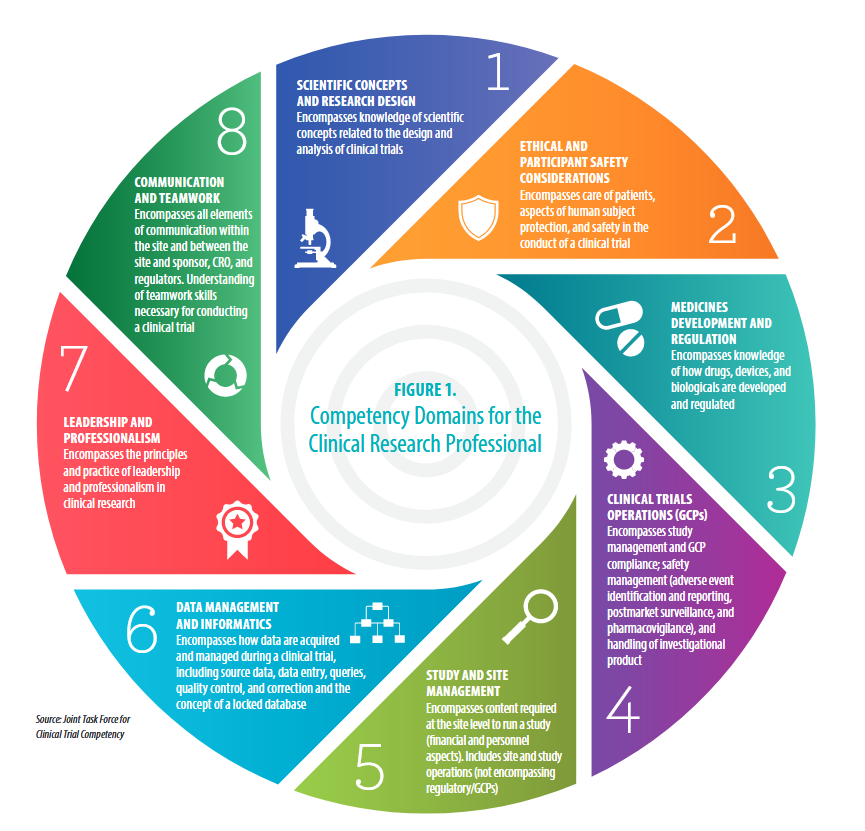
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
Submitting research projects for IRB review can be a stressful experience. Many people wonder what steps they can take to minimize requests for modifications. This presentation will go over the common reasons for these determinations. In addition, this talk will address concrete ways researchers can prevent or minimize these types of issues, and provide advice on how to respond to the IRB. Finally, this talk will go over steps researchers can take to streamline the submission process.
Speaker:
Rachel Olech
Senior Regulatory Specialist, CCTS
Competency Area:
#2: Ethical and Participant Safety Considerations
Register for Zoom login details
Date posted
Oct 29, 2024
Date updated
Oct 29, 2024