“IND Regulations”
Best Practice Hour Seminar Series
February 18, 2025
12:00 PM - 1:00 PM
Location
Virtual via Zoom
Calendar
Download iCal File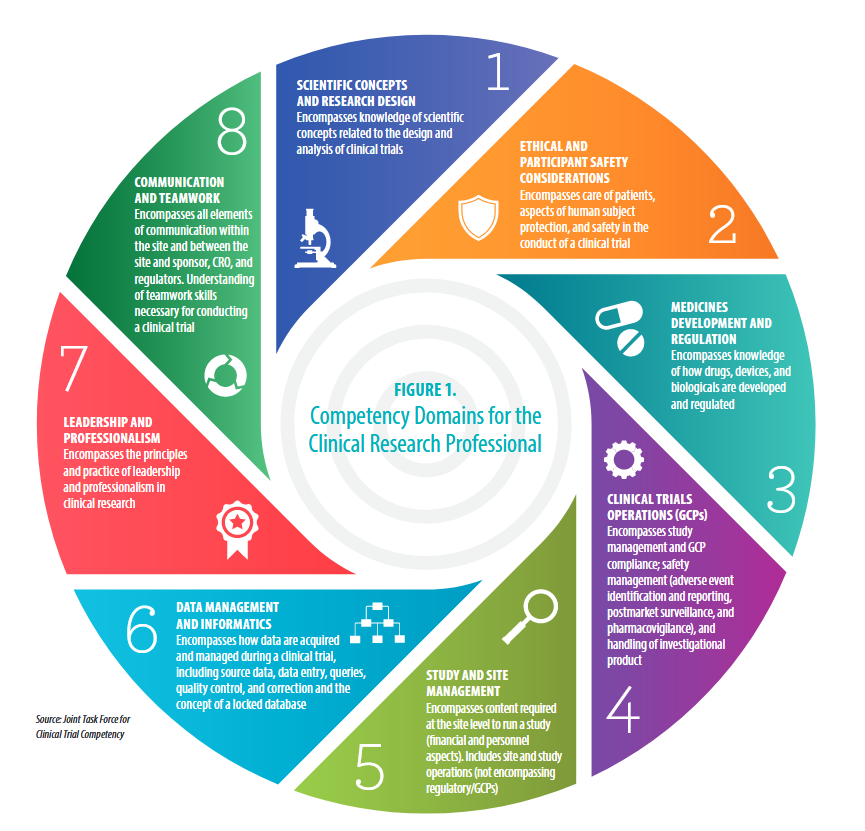
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
This seminar will cover the IND submission process, specifically focusing on:
- Investigator initiated IND
- Single patient (emergency use) IND
- Requirements after IND is approved for use
Speaker:
Darlene Kitterman, MBA
Director, Clinical Trials Office
University of Illinois Cancer Center
Competency Area:
#3: Medicine Development & Regulation
Register for Zoom login details
Date posted
Oct 7, 2024
Date updated
Nov 14, 2024