“UIC Clinical Trials Review Committees: What, When, & How?”
Best Practice Hour Seminar Series
November 19, 2024
12:00 PM - 1:00 PM
Location
Virtual via Zoom
Calendar
Download iCal File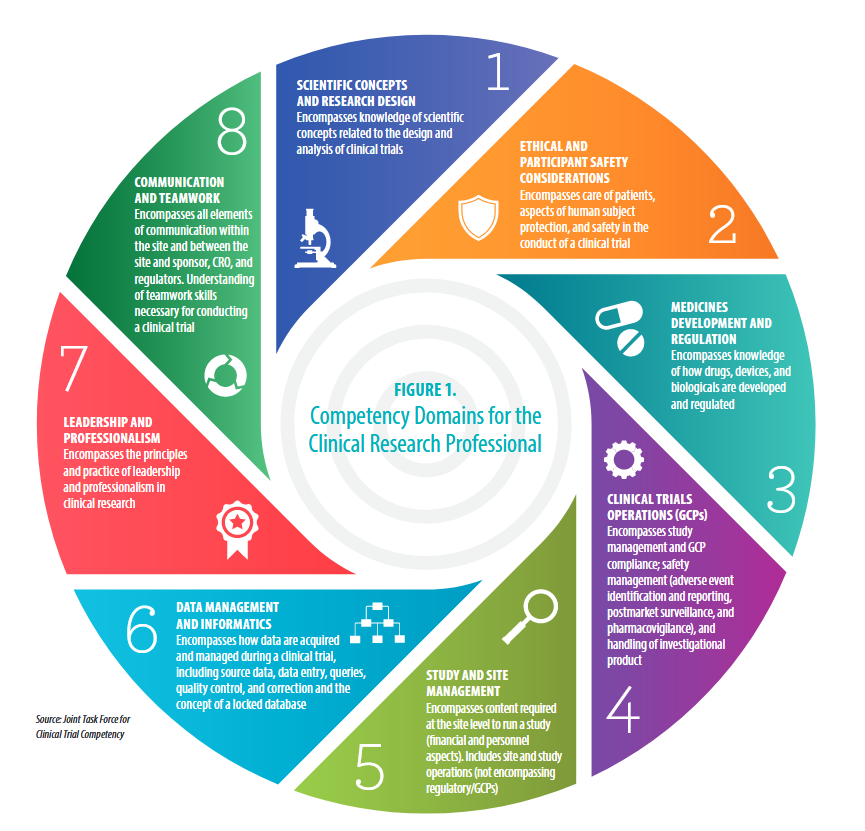
https://clic-ctsa.org/publications/ecrptq-competencies
Description:
This seminar will describe the potential review processes required to obtain regulatory approval for clinical research conducted at UIC, when these reviews are required, and how to submit projects for review.
Speaker:
Darlene Kitterman, MBA
Director, Clinical Trials Office
University of Illinois Cancer Center
Competency Area:
#4: Clinical Trials Operations
Register for Zoom login details
Date posted
Jul 18, 2024
Date updated
Nov 14, 2024